We see new ways to deliver drugs
Ripple is a clinical stage company focused on improving ophthalmic therapeutics with controllable, sustained drug delivery.
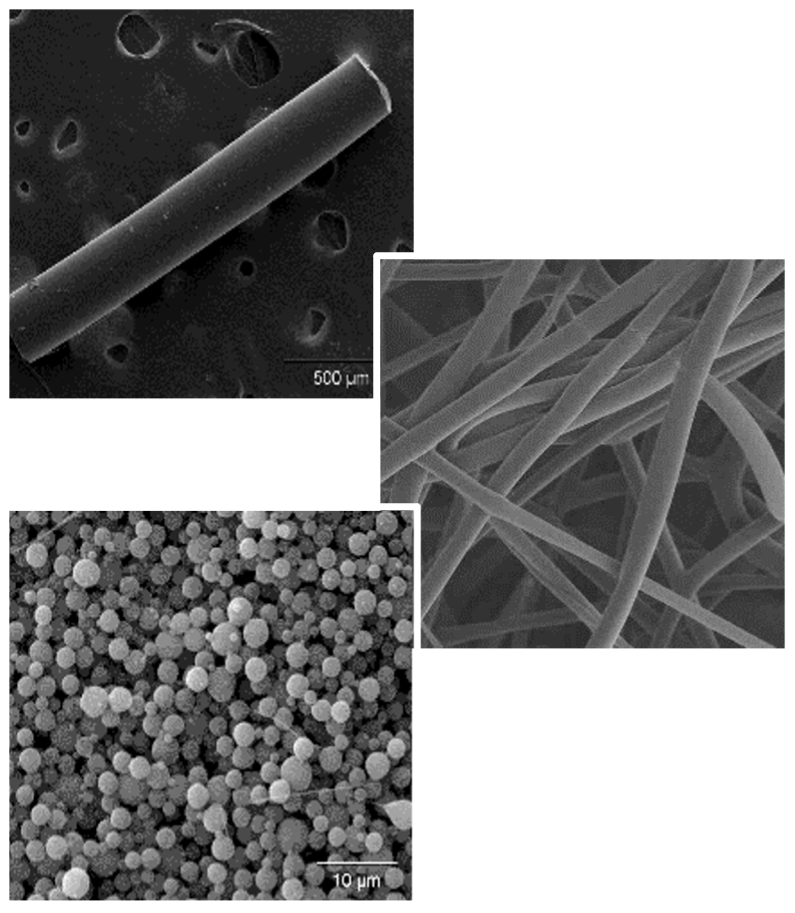
Our patented Epidel® platform is based on a discovery that drugs can be chemically engineered into controlled release pharmaceuticals without the use of polymers. These proprietary prodrugs undergo surface erosion to enable a constant sustained drug release and are highly engineerable to tailor drug dose and duration to the specific indication.
With an extended duration of therapeutic benefit, our technology eliminates the need for daily drops or monthly injections, reducing the treatment burden for patients.
Ripple’s Discovery
Epidel™ is founded on a discovery that drugs can be designed to deliver themselves without the need for polymers. Epidel™ prodrug materials are readily manufactured into implantable forms like cylinders and microspheres. Drug dissolves from the surface of the implant via surface erosion in a highly predictable manner: dose is dictated by implant surface area; duration by implant diameter. The result is an ability to finely tune pharmacokinetics to meet the needs of a target indication with precision control of drug dosing without the bulk added by a polymer.
In the true spirit of invention, Epidel was borne out of constraint. In the mid-1990s, the Controlled Release Field was focused on conjugating drugs into polymer backbones as a way of controlling drug release. While the science was conceptually appealing, the lack of reproducible manufacturing and the complexity of a polydisperse polymeric breakdown profile limited the translation into approval products.
In our Toronto laboratories, these constraints inspired a minimalistic approach to re-think how controlled drug delivery could be achieved. A novel prodrug approach was discovered which provided a simple, highly predictable, and tunable way to achieve sustained drug release without the use of polymers or excipients.
No polymers. ONLY PRODRUGS.
Engineered Prodrugs with a Material Advantage
- Inherent processability
- High drug loading
- No inflammatory byproducts
- Surface mediated drug release
- No polymer residue
RIPPLE timeline
Ripple Therapeutics was founded in 2020 (technology spin out from Interface Biologics (IBI) after the successful sale of its surface modification assets to Evonik (EVR-ETK) in 2019).
2017 - 2019
Discover prodrugs can be engineered into implants without polymers. Establish focus in ophthalmology. Preclinical data confirms safety, efficacy and duration of Dex IVT implant.

2020
$15M Series A Financing; Licensing Agreement signed with TOI.

2021
First patients with RVO and DME treated in Ripple-1 with Dex IVT implant; Key patents granted; Pipeline expansion into Glaucoma.
2022
Ripple-1 Clinical Trial demonstrates safety, efficacy and duration of Dex IVT implant; Glaucoma implant preclinical data confirms safety & efficacy.

2023
Glaucoma preclinical repeat dosing studies establish long term safety after a second implant is administered.

2024 - 2025
Ripple announces strategic partner agreements with AbbVie, Glaukos & Bausch + Lomb. Expands pipeline to include Dry AMD/GA.
PartnersHIPS
In the Fall of 2024, Ripple Therapeutics announced two partnership agreements.
The first is a collaboration and option-to-license agreement with AbbVie (NYSE: ABBV) to develop RTC-620, a next generation, fully biodegradable, sustained release drug delivery intracameral implant with repeat dosing capabilities to reduce intraocular pressure (IOP) in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT). This collaboration leverages AbbVie's expertise in eye care and Ripple’s innovative drug delivery platform. Under terms of the agreement, Ripple leads preclinical development of RTC-620 and upon exercise of the option, AbbVie will lead the clinical and commercialization activities. Ripple received an upfront payment of $21.8 million from AbbVie and is eligible to receive up to $290 million in aggregate option fees and milestones, as well as tiered royalties on net sales.
Additionally, Ripple announced evaluation and licensing agreements with Glaukos Corporation (NYSE: GKOS), and in 2025 with Bausch + Lomb (NYSE/TSX: BLCO).
About us
Our leadership team has experience in managing both small and large public and private companies through various stages of growth and financing strategies (venture capital, M&A transactions, IPOs, PIPEs). They successfully sold the surface modification business of Interface Biologics to Evonik (ETR:EVK) in 2019 and founded Ripple Therapeutics in January of 2020.
Our team is made up of a high calibre group of PhD and MSc Chemists, Engineers and Material Scientists.

