Ripple Therapeutics Presents at ARVO 2024
Ocular tolerability and IOP-lowering evaluation of the RTC-620 intracameral implant in normotensive beagle dogs
Ike Iqbal K Ahmed, Kyle Battiston, Shadi Taghavi, Mahta Massoud, Eamon Kelly, Hans Fischer, Dimitra Louka, Matthew Statham, Jonathan Day, Adam Daley, Ian Parrag, Wendy Naimark
IBE-814 IVT Implants reduce treatment burden in subjects with DME and RVO due to sustained-release of dexamethasone: An analysis of the First-in-Human Phase 2 RIPPLE-1 trial
Kelli Wootton, Gillian Mackey, Madeline Simpson, Joseph Reiz, Wendy Naimark

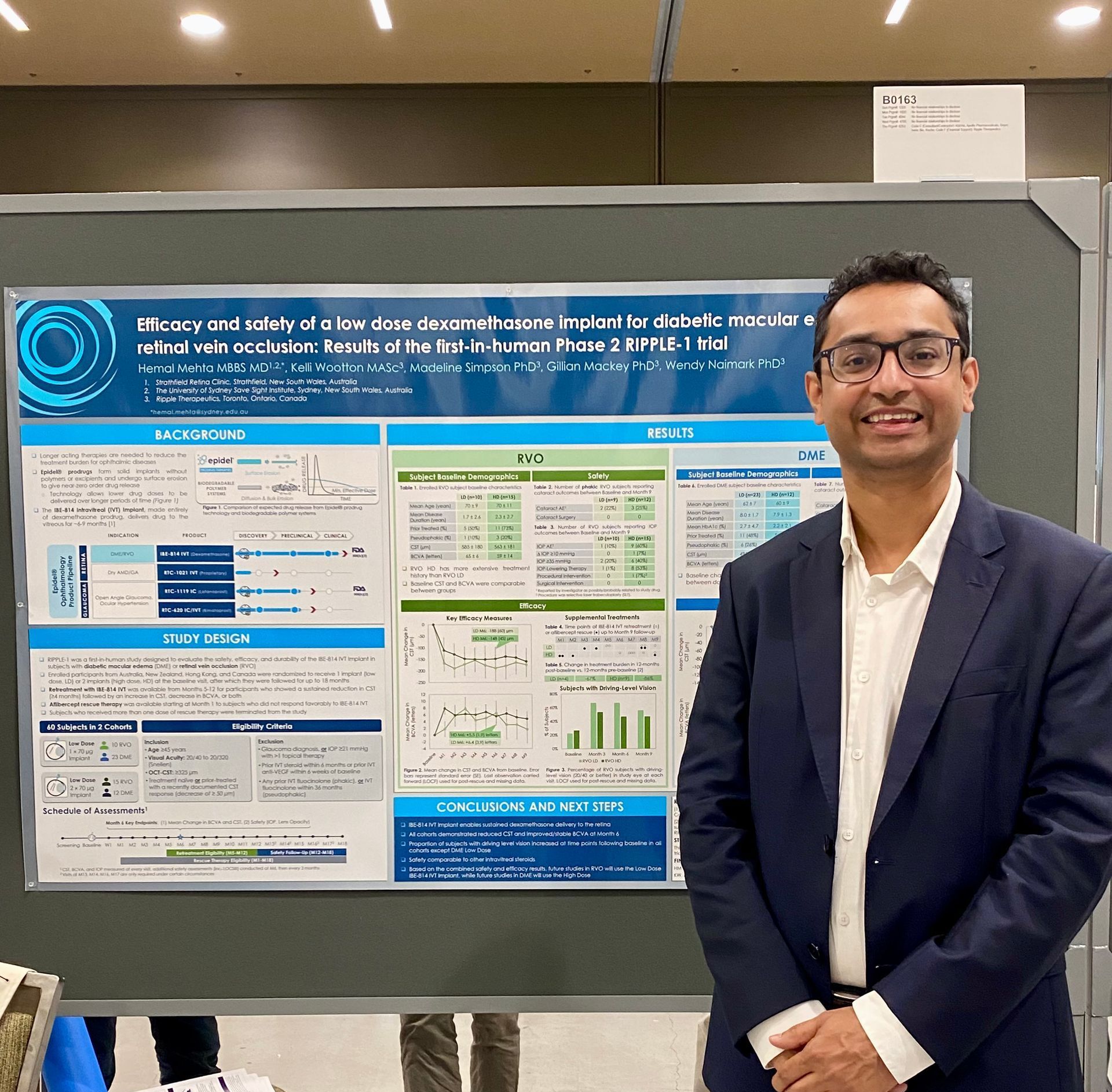
Efficacy and safety of a low dose dexamethasone implant for diabetic macular edema and retinal vein occlusion: Results of the First-In-Human Phase 2 RIPPLE-1 trial
Hemal Mehta, Kelli Wootton, Madeline Simpson, Gillian Mackey, Wendy Naimark
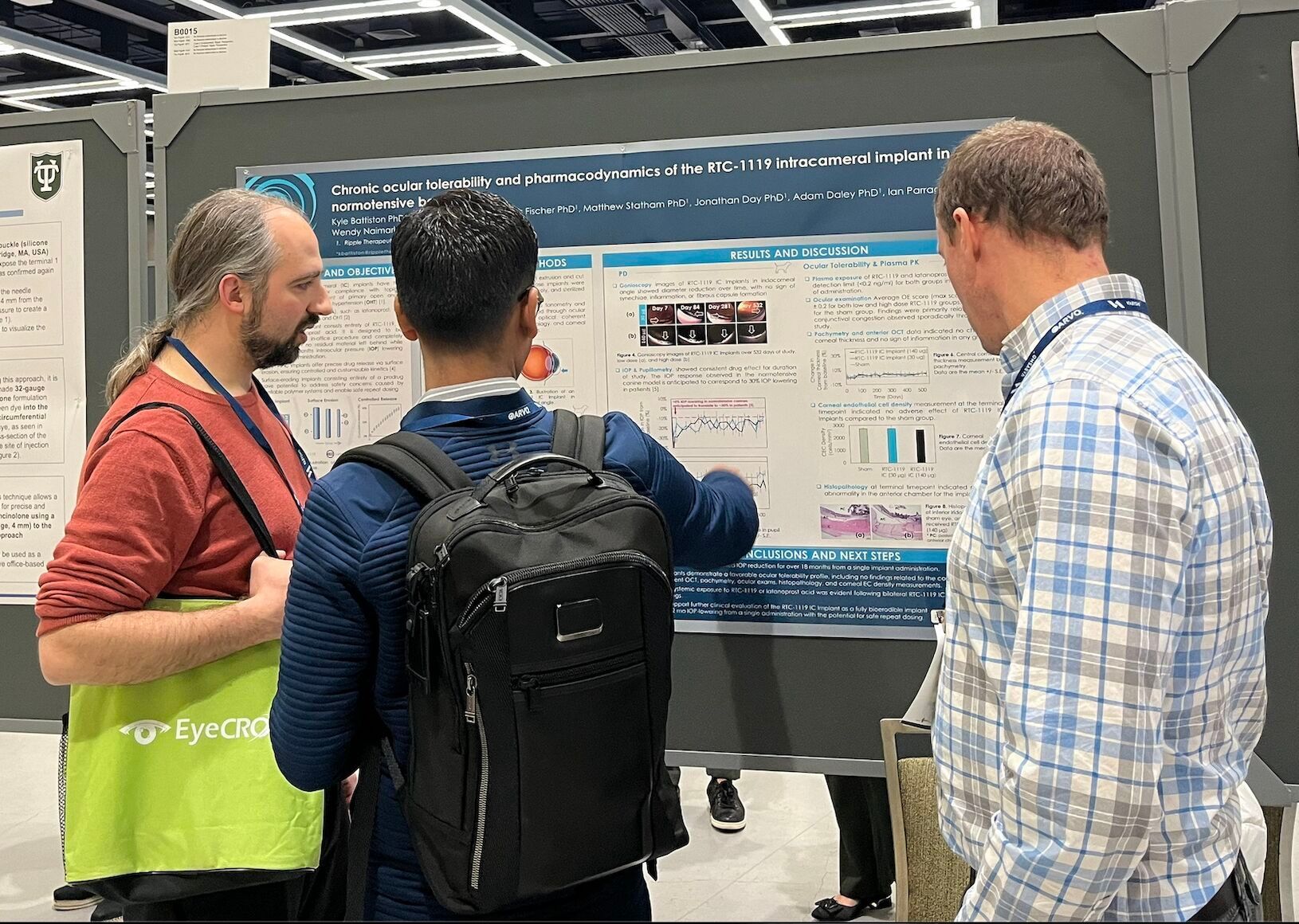
Chronic ocular tolerability and pharmacodynamics of the RTC-1119 intracameral implant in normotensive beagle dogs
Kyle Battiston, Shadi Taghavi, Hans Fischer, Matthew Statham, Jonathan Day, Emily Baldwin, Adam Daley, Ian Parrag, Wendy Naimark
TORONTO, CANADA / November 25, 2025 - Ripple Therapeutics Corporation, a clinical stage company focused on improving ophthalmic therapeutics with controllable sustained delivery implants, is pleased to announce that, earlier this year, it entered into an evaluation program and licensing option agreement with an affiliate of Bausch + Lomb Corporation (NYSE/TSX: BLCO), a leading global eye health company dedicated to helping people see better to live better.
Co-Founder and Chief Technology Officer, Wendy Naimark, PhD., recently presented “Overview of the Epidel Prodrug Engineered Sustained Drug Delivery Platform” at PODD: Partnership Opportunities in Drug Delivery. Click to View

